This COVID vaccine can be inhaled [aerosolized] and its being made and tested in Canada,2021!
Will this be sprayed atmospherically without our permission...we must ask

Canadian-Chinese scientists are set to start testing an inhaled COVID-19 vaccine in humans that targets not only the ever-mutating spike protein the pandemic virus uses to grab onto human cells, but two others that aren’t nearly so prone to mutations.
Instead of being injected into the deltoid muscles in the arm, the McMaster University vaccine is delivered via tiny aerosol particles breathed deep into the lungs.
The vaccine is part of a second generation of COVID-19 vaccines and one of two Canadian-made formulas that hope to offer a more robust, and more stable immune response. On Tuesday, Quebec-based Medicago Inc. is due to report results from its Phase 3 trial of its plant-based vaccine that involved some 24,000 people, the last step of testing before possible approval.
The McMaster team’s vaccine is being trialed as a booster for people who have received two doses of an mRNA vaccine, such as Pfizer-BioNTech or Moderna. The original idea, early in the vaccine race, was to offer it as a standalone, “but as time has gone on, virtually everybody has now had the opportunity to get an mRNA vaccine,” said Fiona Smaill, a professor of pathology and molecular medicine at McMaster who is leading the human trial.
But with SARS-CoV-2 set to linger for months, perhaps years, and more booster doses likely to follow, it’s still important to test new vaccines that work in different ways, Smaill said.
A potential godsend for the needle phobic, McMaster’s formula is administered through a jet nebulizer that generates a fine, misty solution, “with particles that are so tiny they’re breathed right down deep into the lung,” Smaill said. The delivery route is built on two decades of research on a tuberculosis vaccine led by McMaster professor Zhou Xing.
Once inside the lungs, the vaccine is designed to deliver a local “mucosal” response, right at the site where the virus enters the body. The aim is a broader type of immune response than can be achieved just by injecting into the muscles of the upper arm, including a more potent T cell response, the researchers said. T cells find and destroy infected cells.
The vaccine uses strains of a weakened adenovirus, a family of viruses that cause the common cold. Two are being tested on 30 healthy volunteers — a vaccine using a human adenovirus, the other a chimpanzee adenovirus.
In addition to the spike protein that peppers the outside of SARS-CoV-2 and that the virus uses to slip inside human cells, the vaccine targets two cellular proteins inside the virus that it needs to grow and spread. “Mutations would be rarely seen in those proteins,” Smaill said.
“If you can generate that local immune response in the lung,” it may prove better to boost with an inhaled vaccine, she said. Canada has also shown that mixing vaccines can trigger a stronger response. “For a number of reasons, this has the promise of being a more effective way to boost,” Smaill said.
Still, human trials are just beginning. Medicago has been submitting data to Health Canada as part of a rolling submission. The federal government has a contract to purchase 20 million doses. Medicago is also planning a study early in 2022 testing its vaccine as a booster.
Omicron has “supercharged ” the issue of boosters. If early anecdotal reports suggesting Omicron causes milder infections pan out, “I think we’re going to be okay,” Smaill said. But until more data are available, “we’re all guessing what’s actually going to happen.”
“We seem to be doing better, this time around, than we were a year ago. We’ve got vaccines. We’ve got an understanding of what the major risks are in terms of transmission. So we’ve made progress. How do we keep on, without ever going back? I think that’s really what I’m looking for.”
Zhou Xing: Does This McMaster University
Vaccine Developer have Connections to the
CCP?
Author: Himalaya Toronto Maple Leaf – Wenjin
Translator: Himalaya Toronto Maple Leaf – Liberte

According to a March 8 report on McMaster University’s website, Zhou Xing, a tenured professor in the Department of Pathology and Molecular Medicine at the university’s Centre for Immunology Research, has led a team to develop a new vaccine for the CCP-virus (Covid-19). What is the apparent relationship between Zhou Xing and the Chinese Communist Party (CCP), the originator of the epidemic?
On October 18, 2011, the website of West China Hospital of Sichuan University published the article: “Professor Zhou Xing, a Canadian immunologist from McMaster University, was appointed as a visiting professor in the Department of Respiratory Medicine of our hospital”

On October 8, 2013, the website of the Chinese Academy of Sciences reprinted an article by Xinhua: “Scientists Develop New Tuberculosis Vaccine” 《科学家开发新型结核病疫苗》, in which they mention Zhou Xing’s interview with Xinhua News Agency, and where they state that they created a new tuberculosis vaccine using a genetically modified cold virus.
Zhou Xing is a visiting professor at Suining Central Hospital. On November 15, 2019, before the outbreak of the CCP-virus, the website of Suining Central Hospital published a report entitled: “Professor Zhou Xing of McMaster University in Canada, visited Suining Central Hospital” 《加拿大麦克马斯特大学邢周教授访问遂宁市中心医院》. The topic of Zhou Xing’s lecture was “Human Vaccination Program – Status and Challenges.”.

On September 2, 2020, the Free Times’ article: “Development of a Chinese-Russian Vu Lung Vaccine Using a Cold Virus Raises Doubts” mentioned that Zhou Xing had worked with Cansino Bio in 2011 to develop their first Ad5-based vaccine against tuberculosis. His team is now developing an inhaled vaccine for the Ad5 virus.
Cansino Bio Company was founded in 2009 in Tianjin by Yu Xuefeng, Zhu Tao, Qiu Dongxu and Helen Mao Huihua. It has very strong connections with the Academy of Military Medical Science of the Chinese People’s Liberation Army (AMMS), which is the highest-level research institute of the People’s Liberation Army (PLA) in China.
The description on Wikipedia states that “The adenovirus vector recombinant Ebola virus vaccine developed by Cansino Biologics and the Academy of Military Medical Science of the Chinese People’s Liberation Army (AMMS) was approved for registration by the China Food and Drug Administration in October 2017.”
“In April 2020, Consino Biologics developed a novel coronavirus vaccine with a recombinant adenovirus vector in collaboration with Academy of Military Medical Science of the Chinese People’s Liberation Army (AMMS).”
“On February 25, 2021, the National Medical Products Administration announced the conditional approval of the marketing registration application of a COVID-19 vaccine (Vero cells) from China National Pharmaceutical Group Co, Wuhan Institute of Biological Products Co. as well as the recombinant new coronavirus vaccine (adenovirus vector type 5/Ad5-nCoV) from Canosino Biologics. The latter is a novel coronavirus vaccine developed by Chen Wei’s team and is the only vaccine that can be administered as a single dose among the novel coronavirus vaccines currently approved for marketing in China.”

The website of Canada’s McMaster University shows that Zhou Xing earned his PhD in immunology at the university in 1993, which generally requires five years to complete. It is doubtful that an ordinary Chinese person, without connections, could go abroad for a PhD back in 1993. Zhou Xing, who has been working with Cansino Bio, a biologics company with a background in the PLA, appears to have been deeply involved in the Communist Party’s projects, since he has been working with the company since 2011. Zhou Xing has also appeared to have been involved in various domestic collaborations and has been interviewed by the CCP’s mouthpiece Xinhua News Agency.
The timing of Zhou Xing’s appearance in Canada, and his collaborations with CCP-linked entities in China, at the same time when the CCP appears to have been playing a strategic game in order to prepare the “perfect crime” appears to be too much of a coincidence. On the one hand, scientists like Xing often return to CCP China to cooperate with domestic schools, hospitals and companies, so that their research results can be used by the CCP, and on the other hand there is at least circumstantial evidence that they carry out specific work such as the development of Chinese viral vaccines according to the CCP’s strategy of implementing targeted biological weapons warfare. Through the brainwashing of the CCP media, Western Mainstream Media and Big Tech (Social Media) propaganda, Canadians are fooled into thinking that the end of the Pandemic is near, not understanding that the CCP is leading them into a vaccine trap.
Reference:
[1] 中共军方背景公司 与加国共研CCP病毒疫苗-GNews
[2] 康希诺生物高管参与中共“千人计划”-GNews
[3] Chinese vaccine company executives worked in program now targeted by Western intelligence agencies
[4] Scientists see downsides to top COVID-19 vaccines from Russia, China
[5] Canadian researchers develop faster, more accurate COVID-19 antibody test
[6] 加拿大特鲁多政府为何只青睐产自中共国的疫苗-GNews
[7] 四川大学华西医院-百度百科
[8] 华西医院有多牛?
[9] 康希诺生物– Wikipedia
[10] PLA Academy of Military Science-Wikipedia
Immunological considerations for COVID-19 vaccine strategies
Nature Reviews Immunology volume 20, pages615–632 (2020)Cite this article
302k Accesses
427 Citations
743 Altmetric
Abstract
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the most formidable challenge to humanity in a century. It is widely believed that prepandemic normalcy will never return until a safe and effective vaccine strategy becomes available and a global vaccination programme is implemented successfully. Here, we discuss the immunological principles that need to be taken into consideration in the development of COVID-19 vaccine strategies. On the basis of these principles, we examine the current COVID-19 vaccine candidates, their strengths and potential shortfalls, and make inferences about their chances of success. Finally, we discuss the scientific and practical challenges that will be faced in the process of developing a successful vaccine and the ways in which COVID-19 vaccine strategies may evolve over the next few years.
Introduction
The coronavirus disease 2019 (COVID-19) outbreak was first reported in Wuhan, China, in late 2019 and, at the time of writing this article, has since spread to 216 countries and territories1. It has brought the world to a standstill. The respiratory viral pathogen severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected at least 20.1 million individuals and killed more than 737,000 people globally, and counting1. Although physical-distancing and other transmission-mitigation strategies implemented in most countries during the current pandemic have prevented most citizens from being infected, these strategies will paradoxically leave them without immunity to SARS-CoV-2 and thus susceptible to additional waves of infection. Health-care workers, seniors and those with underlying health conditions are at particularly high risk2,3,4. It is widely accepted that the world will not return to its prepandemic normalcy until safe and effective vaccines become available and a global vaccination programme is successfully implemented5.
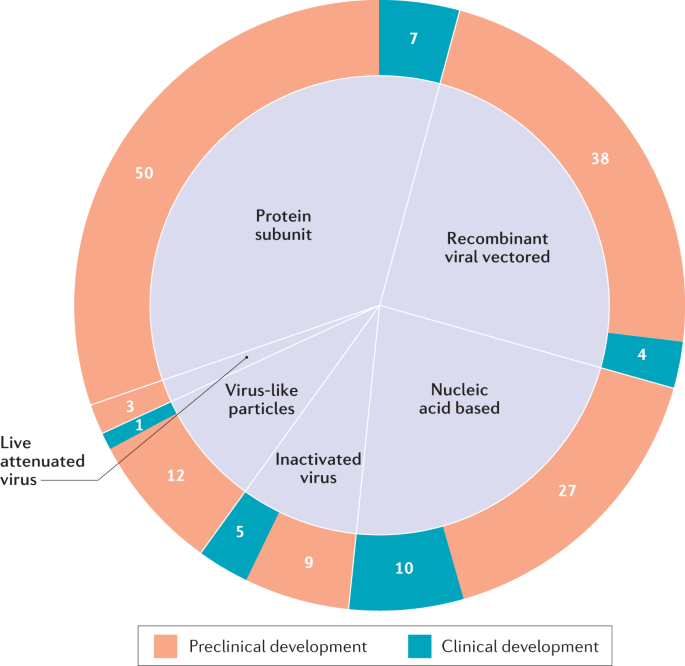
As COVID-19 is new to humankind and the nature of protective immune responses is poorly understood, it is unclear which vaccine strategies will be most successful. Therefore, it is imperative to develop various vaccine platforms and strategies in parallel. Indeed, since the outbreak began, researchers around the world have been racing to develop COVID-19 vaccines, with at least 166 vaccine candidates currently in preclinical and clinical development5 (Fig. 1). To meet the urgent need for a vaccine, a new pandemic vaccine development paradigm has been proposed that compresses the development timeline from 10–15 years to 1–2 years6. However, there remains a lack of clarity as to what may constitute a safe and immunologically effective COVID-19 vaccine strategy, how to define successful end points in vaccine efficacy testing and what to expect from the global vaccine effort over the next few years. This Review outlines the guiding immunological principles for the design of COVID-19 vaccine strategies and analyses the current COVID-19 vaccine landscape and the challenges ahead.
The six major types of candidate vaccine for coronavirus disease 2019 (COVID-19) are illustrated (live attenuated virus, recombinant viral vectored, inactivated virus, protein subunit, virus-like particles and nucleic acid based), showing the number of candidate vaccines that are currently under clinical and preclinical development. The nucleic acid-based platform includes both mRNA vaccines (6 clinical and 16 preclinical) and plasmid DNA vaccines (4 clinical and 11 preclinical). Data obtained from ref.5.
Natural and vaccine-induced immunity
Although much remains to be understood regarding the immune response to SARS-CoV-2, and vaccine-induced protective immunity may differ from natural immunity owing to the immune-evasion strategies of the virus, improved understanding of the natural immune response will be instrumental in developing effective vaccine and therapeutic strategies. It is particularly relevant to understand the difference in immune responses between asymptomatic, mild and severe cases and at early and late stages of infection, and to understand why seniors are particularly susceptible to COVID-19, whereas the young are better protected. It is estimated that 40–75% of infections may be mild or asymptomatic7,8 and asymptomatic individuals may have a significantly longer duration of viral shedding than their symptomatic counterparts9. Furthermore, that asymptomatic and mildly ill individuals seem to develop low levels of antibody-mediated immunity has important implications for understanding herd immunity.
The initial site of infection of SARS-CoV-2 is the respiratory tract10,11. On entry, SARS-CoV-2 interacts with the angiotensin-converting enzyme 2 (ACE2) receptor on bronchial and alveolar epithelial cells through its spike (S) protein receptor-binding domain (RBD), which is subsequently primed by a specific cellular serine protease, transmembrane protease serine 2 (TMPRSS2), to gain entry12,13. Analysis of transcripts encoding ACE2 and TMPRSS2 by single-cell RNA sequencing has shown that these transcripts are co-expressed in various cell types10,11, and from autopsy studies SARS-CoV-2 can be detected in multiple organs, including the lungs, pharynx, heart, liver, brain and kidneys14.
Innate immune responses
Emerging evidence suggests that the immune response to SARS-CoV-2 is similar in several aspects to the response to SARS-CoV or Middle East respiratory syndrome coronavirus (MERS-CoV), the two coronaviruses responsible for the 2002–2004 SARS outbreak and the 2012 MERS outbreak that originated in China and Saudi Arabia, respectively15,16,17. Like SARS-CoV and MERS-CoV, SARS-CoV-2 suppresses activation of the innate immune system, including dendritic cells18,19, and dampens antiviral type I and type III interferon responses20. This ability of SARS-CoV-2 to subvert the innate immune response may explain the protracted incubation or presymptomatic period of 2–12 days for COVID-19 relative to the 1–4-day incubation period for influenza16. Thus, uncontrolled SARS-CoV-2 replication in the early phase of infection resulting from innate immune suppression probably underpins the ensuing dysregulated inflammatory responses16,21, particularly in severe cases of COVID-19. Such cases are characterized by markedly increased numbers of inflammatory monocytes and neutrophils in blood20,22,23 and CD14+CD16+ monocyte-derived macrophages in the airway20,24, and increased systemic levels of inflammatory cytokines and chemokines20,22,23. A failure to accomplish early control of SARS-CoV-2 infection in the respiratory tract likely results in high viral burden and dysregulated, potentially lethal, inflammatory responses and immunopathology, including acute respiratory distress syndrome. For this reason, seniors and those with co-morbidities may be particularly prone to COVID-19 owing to immunosenescence and their propensity to mount exaggerated inflammatory responses25,26,27. Besides the consideration of vaccine-induced adaptive immunity discussed later, inclusion of the recently emerged concept of trained immunity (Box 1) in COVID-19 vaccine design might further bolster protection, particularly in the early phases of infection.
Antibody responses
IgM and IgG antibodies to SARS-CoV-2 are detectable within 1–2 weeks after the onset of symptoms in most infected individuals28. Although the relationship between neutralizing antibodies and antigen-specific T cells and disease severity and clinical outcomes remains to be understood, high levels of neutralizing antibodies have been observed in convalescent individuals29, which correlate with T cell responses, particularly those of CD4+ T cells30, and seem to offer some benefits in studies of treatment with convalescent plasma31. Recent studies indicate that the magnitude of neutralizing antibody responses is positively correlated with disease severity32. Thus, whereas antibody responses wane within weeks after infection in most people infected with SARS-CoV-2 (ref.32), which is a feature of antibody responses to other ‘common cold’ coronaviruses17, the magnitude of the neutralizing antibody response in asymptomatic individuals is not only smaller but also decreases faster than in symptomatic individuals9.
The major target of neutralizing antibodies to coronaviruses is the S protein, which is composed of S1 and S2 domains. S1 is membrane distal and contains the RBD that binds to the cellular receptor ACE2. S2 is membrane proximal and has a role in membrane fusion33. The S proteins of SARS-CoV and SARS-CoV-2 are 88% identical and both bind to ACE2 with high affinity33. Certain monoclonal and polyclonal antibodies raised to the S protein of SARS-CoV can cross-neutralize SARS-CoV-2 (refs33,34). Antibodies that bind to the S1 RBD block its interaction with ACE2, whereas those that bind to other regions of S1 and S2 can inhibit conformational change of the S protein and block membrane fusion, respectively35,36,37.
During natural immune responses to SARS-CoV-2, high titres of antibodies are also generated against nucleoprotein (N) — the most abundant viral protein38,39,40. Although antibodies to N are unlikely to neutralize the virus, they have been reported to provide protection against mouse hepatitis virus, a coronavirus of mice. Notably, these antibodies were IgG2a, indicating that they may exert protection through Fc-mediated effector functions rather than direct virus neutralization41,42. Somewhat unusually, several studies have reported that IgA responses to S protein peak earlier than IgM responses and are more pronounced, which makes IgA a potentially attractive target for antibody-based diagnostic assays43,44. The mechanistic basis of this early induction of S-specific IgA is not yet clear.
We do not yet understand the durability of the antibody responses to SARS-CoV-2. However, previous longitudinal studies of patients with SARS-CoV infection reported substantial waning of neutralizing antibody titres between 1 year and 2 years after infection45,46. This is consistent with classical studies showing a relatively rapid waning of antibodies to the seasonal coronavirus 229E47. There are currently no immune correlates of protection for SARS-CoV-2 or other human coroanviruses. Thus, it is unclear what titre of neutralizing antibodies is sufficient to confer protection against infection. Establishing such correlates will be essential to guide the development of effective COVID-19 vaccines.
T cell-mediated immunity
Whereas the current successful human antiviral vaccines, such as influenza and measles vaccines, depend largely on the induction of antibody responses, emerging evidence suggests the requirement of both antibody-mediated and T cell-mediated immunity for effective protection against SARS-CoV-2 (refs17,27). It is well known that CD4+ T cell help is important for optimal antibody responses and for CD8+ T cell activation in host defence48. Furthermore, if neutralizing antibody-mediated protection is incomplete, cytotoxic CD8+ T cells are crucial for viral clearance49. One study found that among people who had recovered from COVID-19, 100% had S protein-specific CD4+ T cells in the circulation and 70% had S protein-specific CD8+ T cells in the circulation30, and preclinical studies show a protective role of T cells in host defence against SARS-CoV50.
The 2–12-day incubation or presymptomatic period of SARS-CoV-2 infection is associated not only with virus-mediated innate immune suppression but also with delayed activation of T cells, particularly CD8+ T cells18,19, as is the case for SARS and MERS. People who have recovered from COVID-19 seem to have high levels of both neutralizing antibodies and T cells, and, compared with severe cases, milder cases of COVID-19 have greater numbers of memory CD8+ T cells in the respiratory tract24,29,30. Evidence suggests that the induction of such lung tissue-resident memory T cells (TRM cells) will depend on the route of vaccination. Respiratory mucosal vaccination induces strong lung TRM cell responses, whereas parenteral vaccination fails to do so51,52,53. Experimentally, the airway TRM cells elicited by respiratory mucosal vaccination offered robust protection against SARS-CoV infection54.
The T helper cell (TH cell) phenotype of vaccine-induced T cells is also relevant to the protection they mediate. Less severe cases of SARS were associated with accelerated induction of a TH1 cell response55, whereas TH2 cell responses have been associated with enhancement of lung disease following infection in hosts parenterally vaccinated with inactivated SARS-CoV viral vaccines56,57. Thus, COVID-19 vaccine-induced TRM cells should have a TH1 cell-like phenotype.
These lines of evidence, together with data suggesting that T cell-mediated immunity generally is a more reliable correlate of vaccine protection than antibody titres in seniors26, strongly support the inclusion of T cell responses in COVID-19 vaccine design17,27.
Pre-existing cross-reactive immunity
Emerging evidence indicates that CD4+ T cells in 35% of healthy individuals not exposed to SARS-CoV-2 recognize the SARS-CoV-2 S protein and that CD4+ T cells in 40–60% of unexposed individuals are reactive to SARS-CoV-2 proteins other than S protein30,58. This indicates that there is cross-reactivity between CD4+ T cells specific for SARS-CoV-2 and CD4+ T cells specific for human common cold coronaviruses, SARS-CoV and animal betacoronaviruses17,59,60,61. There are four human coronaviruses — 229E, NL63, OC43 and HKU1 — that account for ~15% of common colds in humans17. Adults may be infected with one of these on average every 2–3 years, such that there could be a degree of pre-existing cross-reactive immunity to SARS-CoV-2 antigens in these people, which offers a potential explanation for differing susceptibility to SARS-CoV-2 infection. In addition to understanding the relationship between such pre-existing immunity to human coronaviruses and host defence against SARS-CoV-2, it will also be important to consider the contribution of COVID-19 vaccine-boosted cross-reactive immune responses to vaccine-induced protective immunity.
Importantly, whereas CD4+ T cells from patients with COVID-19 equally recognize the S1 and S2 subunits of SARS-CoV-2, cross-reactive CD4+ T cells from unexposed individuals recognize the S2 subunit58. CD4+ T cells from patients with COVID-19 cross-react strongly with S2 subunits of the human coronaviruses OC43 and 229E. More than 90% of tested healthy adults also have IgG antibodies specific for all four human common cold coronaviruses17. However, similarly to antibody responses to SARS-CoV and SARS-CoV-2, antibody responses to human coronaviruses wane rapidly within months after infection. Therefore, control of reinfection with human coronaviruses seems mainly to be antibody independent but T cell dependent17.
As coronavirus cross-reactive T cells can be specific for both structural and non-structural viral proteins58,61, the extent of vaccine-boosted cross-reactive T cell responses induced by most protein subunit and recombinant viral-vectored COVID-19 vaccines, which are currently based only on the S protein, will be different from those boosted by multivalent COVID-19 vaccines such as those based on inactivated SARS-CoV-2 virus. One exception could be the use of live attenuated SARS-CoV-2 vaccines as the pre-existing cross-reactive immunity may limit the potency of such vaccines. Finally, it is noteworthy that the significant presence of cross-reactive immunity in some individuals calls for consideration of stratifying clinical trial participants receiving candidate COVID-19 vaccines according to their status of pre-existing coronavirus immunity.
Antibody-dependent enhancement of disease
A potential barrier to the development of safe and efficacious COVID-19 vaccines (Box 2) is the risk that insufficient titres of neutralizing antibodies might trigger antibody-dependent enhancement (ADE) of disease. ADE is most classically associated with dengue virus, whereby cross-reactive but subneutralizing concentrations of antibodies to one virus serotype enhance infection with another serotype in Fcγ receptor (FcγR)-bearing cells, including macrophages62. A common property among viruses that cause ADE is an ability to replicate in macrophages and/or cause them to respond abnormally. Although macrophages do not seem to be a major target of SARS-CoV-2 infection, and the expression of ACE2 on different monocyte and macrophage populations is highly variable, previous data regarding SARS-CoV suggest that FcγRs can facilitate uptake of the virus into macrophages and B cells21,63. Cytokine profiles from patients infected with SARS-CoV-2 resemble those in macrophage activation syndrome and are characterized by high levels of inflammatory cytokines and chemokines21,64,65,66. Furthermore, patients with symptomatic COVID-19 are reported to produce IgG antibodies with reduced fucosylation levels, which in turn promotes their interaction with activating FcγRIIIa67.
The evidence for ADE in the context of SARS-CoV infection is circumstantial. Correlations between antibody titres and infection severity have been reported, but it is unclear whether high antibody titres contribute to disease or whether severe infections elicit higher antibody titres68. Also, macrophages treated in vitro with serum from patients with SARS had exaggerated inflammatory cytokine profiles69,70.
ADE has been reported in some preclinical animal models vaccinated with experimental SARS-CoV vaccines. Ferrets vaccinated with a modified vaccinia virus Ankara (MVA) vaccine expressing full-length S protein had increased infection and hepatitis following challenge71,72. Antibodies to S protein were reported to induce acute lung injury in experimentally infected macaques on the basis of histological examination69. By contrast, hamsters vaccinated with recombinant, full-length SARS-CoV S protein were protected against infection despite the ability of antibodies to mediate entry of SARS-CoV into B cells through FcγRII (ref.73).
Whether ADE occurs in the context of SARS-CoV-2 infection remains unclear but warrants further investigation, focusing directly on whether antibodies increase disease severity and, if so, characterizing the specific properties of these antibodies. What seems clear is that high levels of neutralizing antibodies can mediate protection. Defining the titres of neutralizing antibodies that are protective, ensuring that COVID-19 vaccines can achieve these titres and avoiding waning of antibodies to subneutralizing levels through frequent boosting will be important to minimize the possibility of ADE. Rationally designed COVID-19 vaccines that omit ADE-inducing, non-neutralizing or weakly neutralizing epitopes in favour of those known to mediate protective responses may also minimize the likelihood of disease enhancement. Finally, there is also evidence from mouse models of dengue virus infection that antiviral T cells help to dampen ADE of disease74. Therefore, a vaccine strategy designed to induce both neutralizing antibodies and robust T cell-mediated immunity may help to mitigate the risk of ADE.
Vaccine design
Vaccine design concerns the selection of antigens, vaccine platforms, and vaccination routes and regimen. The choice of vaccine platform determines the relative immunogenic strength of vaccine-derived viral antigens, whether an immune adjuvant is required and the nature of protective immunity. These attributes also determine the suitability of a vaccine for a particular route of vaccination, and whether a prime–boost vaccination regimen is required to increase vaccine-mediated protective immunity and its durability. Furthermore, the selection of live attenuated viral vaccines or a respiratory mucosal route of vaccination will require more stringent safety testing (Box 2).
Selection of SARS-CoV-2 antigens
The structural proteins present in the infectious virion include S protein, N protein, matrix (M) protein and envelope (E) protein. The N protein coats the large positive-stranded RNA genome, which is encased in a lipid envelope derived from the host cell membrane, into which the other three proteins (S, M and E) are inserted. In the case of SARS-CoV, it has been shown that only antibodies directed to S protein can neutralize the virus and prevent infection75. As a result, all SARS-CoV-2 vaccines in development include at least a portion of the S protein. These may be restricted to only the S1 domain or the RBD.
Non-neutralizing antibodies to both S protein and the other exposed proteins (E and M) are generated. As there is a suspected role of these non-neutralizing antibodies, as well as weakly neutralizing antibodies, in ADE of disease, the inclusion of other structural (N) and/or non-structural proteins as vaccine antigens may help to create a more balanced response involving both humoral and T cell-mediated immunity. These could be highly expressed proteins such as N protein or highly conserved functional proteins that have a crucial role in the viral life cycle. For example, inclusion of viral enzymes such as the RNA-dependent RNA polymerase in a vaccine design may ensure that it targets all emerging variant strains, as these proteins are highly conserved59,76,77, even across other bat-derived coronaviruses that could emerge as a threat to humans in the future.
Vaccine platforms
In general, vaccine platforms are divided into six categories: live attenuated virus, recombinant viral-vectored vaccines that are bioengineered to express target pathogen antigens in vivo, inactivated or killed virus, protein subunit vaccines, virus-like particles (VLPs) and nucleic acid-based (DNA or mRNA) vaccines. In broad terms, vaccines require two components: antigens from the target pathogen that are provided to or generated by the vaccine recipient; and an infection signal (such as a pathogen-associated molecular pattern or damage-associated molecular pattern) that alerts and activates the host immune system. Live attenuated vaccines can naturally provide both of these components, whereas non-viral vaccine platforms can provide the antigens but often require the artificial provision of signals to alert the immune system known as adjuvants. Typically, these non-viral vaccine platforms require multiple vaccinations to induce protective immunity, whereas live virus-based vaccines have the ability to provide ‘one-shot’ immunity. Similarly to non-viral platforms, killed virus vaccines sometimes require the inclusion of an adjuvant and repeated administration for full efficacy78. There are immunological pros and cons to each of these technologies as discussed later (Table 1).
Vaccination routes and regimens
In addition to the careful selection of vaccine antigens and platform, the route of vaccination is an integral consideration of vaccine strategies52,79. This is particularly important for mucosal pathogens such as SARS-CoV-2 and those pathogens against which optimal protection requires not only neutralizing antibodies but also innate and adaptive cellular immunity17,80. The best window of opportunity for SARS-CoV-2 control and clearance is the asymptomatic or presymptomatic period of COVID-19 (2–12 days), which is likely to require all of the immune protective elements to be present within the respiratory mucosa before viral entry16,17,27. The route of vaccination has a crucial role in determining this52,81. Protective IgG antibodies induced by parenteral vaccination readily appear at the respiratory mucosa, this being the primary mechanism by which intramuscular injection of measles or influenza vaccine offers protection in humans. However, this route of vaccination is unable to effectively induce mucosal IgA antibodies or TRM cells in the lungs52,81. By comparison, the respiratory mucosal route of vaccination is adept at inducing antibodies and TRM cells in the respiratory mucosa, as well as macrophage-mediated trained immunity52,54,80,81,82,83,84,85 (Box 1). Inactivated virus, protein subunit and nucleic acid vaccines cannot be administered by the respiratory mucosal route owing to their requirement for potentially unsafe immune adjuvants and repeated delivery (Table 1). By contrast, recombinant viral-vectored vaccines, particularly those using human serotype 5 adenovirus (Ad5) or chimpanzee-derived adenovirus (ChAd), are safe and highly effective for respiratory mucosal vaccination79.
Often, weakly immunogenic vaccines based on inactivated virus, protein subunits, nucleic acids or viral vectors such as Ad26 require a repeated homologous vaccination regimen to be effective. Indeed, most current human vaccines require repeated doses. As it is not yet known which COVID-19 vaccine strategy will be used or for how long the vaccine-induced protection may last in humans, it remains possible that a homologous or heterologous prime–boost vaccination regimen will be required to sustain protection, even with robust stand-alone platforms such as ChAd. The same or a different route may be used for the repeated vaccine delivery.
Major COVID-19 vaccine candidates
As of 31 July 2020, there were 27 vaccine candidates for COVID-19 in clinical evaluation and 139 vaccines in preclinical development5 (Fig. 1). Of the 27 vaccines undergoing clinical evaluation (Table 2), the three lead candidates are viral-vectored and mRNA-based vaccines that entered clinical trials in China, the UK and the USA in mid-March 2020. Clinical trials for the remaining 24 candidates are currently recruiting volunteers, and a couple of other candidates are also about to enter clinical trials (Table 2). Preclinical evaluation of candidate vaccines requires the use of relevant animal models of COVID-19 (Box 3). Conventionally, the safety, immunogenicity and protective efficacy of experimental vaccines are rigorously evaluated and established in animal models first before clinical trials are begun. In the case of pandemic vaccine development, however, the preclinical and clinical stages of vaccine development are compressed and move forwards in parallel.
Box 3 Animal models of COVID-19 for vaccine testing
There is an urgent need to identify suitable animal models for the preclinical evaluation of coronavirus disease 2019 (COVID-19) vaccines182. A large number of animal species have differing degrees of susceptibility to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, depending on the relative binding affinity of the virus to the host angiotensin-converting enzyme 2 (ACE2) receptor or on host protease activities on the S protein183.
Among the animal species tested, ACE2 of rhesus macaques has the greatest binding activity for SARS-CoV-2 (ref.183). Infected macaques shed SARS-CoV-2 from the upper and the lower respiratory tract but they do not develop the same clinical signs and age-dependent disease severity as humans184,185. Cats, ferrets and hamsters are also susceptible to SARS-CoV-2 infection. Notably, natural airborne and contact transmissions of SARS-CoV-2 have been reported in cats and hamsters, respectively, but not in ferrets186. Hamsters, but not cats and ferrets, manifest severe clinical symptoms. Thus, these animal models are differentially capable of recapitulating relevant aspects of COVID-19.
Mouse models are widely used for vaccine testing owing to their affordability and the availability of immunoreagents and transgenic mouse strains. However, the ACE2 of conventional mice does not bind well to SARS-CoV S protein187. Transgenic mice expressing human ACE2 were initially developed and thoroughly characterized for the study of SARS-CoV and have now been shown to support SARS-CoV-2 replication in the lung, and these mice develop interstitial pneumonia similar to humans188. Human ACE2-expressing mice that are further humanized to express human HLA genes and/or to have human immune cells will be useful for studying human immune responses and immunodominant epitopes following vaccination and viral infection with SARS-CoV-2. Beyond animal models, of further relevance to human applications is the ongoing ethical debate regarding intentional challenge of vaccinated young people with SARS-CoV-2.
Live attenuated viral vaccines
Historically, several successful human vaccines, such as measles vaccine and the bacillus Calmette–Guérin (BCG) vaccine for tuberculosis (TB), have been based on attenuated strains of the actual pathogen86, with loss or mutation of virulence genes through in vitro passage. It is now possible to rationally design attenuated virus strains by mutating or deleting virulence genes. These deletion mutants can often replicate to a limited extent in host cells but lose the ability to cause disease in vivo. Coronaviruses have several genes that are not required for replication and that can be deleted, leading to attenuation in vivo. Deletion of various non-structural proteins, as well as of the structural E protein, has been used as a strategy to engineer vaccine strains of several zoonotic and veterinary coronaviruses87,88,89. Deletion of the E protein leads to attenuation and generation of an efficacious vaccine strain87,88, but reversion of the attenuated phenotype has been reported90. Deletion of virulence factors may therefore provide a preferred mechanism of attenuation. For example, deletion of the 2′-O-methylase gene from the SARS-CoV genome removes the ability of the virus to hide its RNA from the host cell proteins MDA5 (also known as IFIH1) and IFIT1, thereby inducing a robust antiviral response in vivo91. Another approach to viral attenuation is known as codon deoptimization, whereby the nucleic acid sequence is modified to use suboptimal codons to encode the wild-type amino acid sequence, which considerably slows the translation of the viral protein during infection. This approach can yield a virus that is highly attenuated in vivo but still able to replicate in vitro if the correct viral protein is selected for deoptimization92,93.
However, the generation of an attenuated strain of a pathogen for use as a vaccine requires demonstration of its inability to revert genetically to become pathogenic (Table 1; Box 2). This is particularly challenging in the case of coronaviruses as they are known to recombine in nature94, and an attenuated vaccine strain could, in theory, recombine with wild coronaviruses to recreate a pathogenic strain. So far, there are only three attenuated SARS-CoV-2 vaccines generated by codon deoptimization under preclinical development, by Mehmet Ali Aydinlar University in Turkey, Codagenix and Serum Institute of India, and Indian Immunologicals Ltd and Griffith University5.
Recombinant viral-vectored vaccines
Recombinant viral-vectored vaccines are built on either a replication-deficient viral backbone or an attenuated replication-competent viral backbone that is bioengineered to express antigens derived from the target pathogen. Although only a couple of viral-vectored vaccines have been approved for human use for the control of infections such as Ebola, this platform has been widely investigated and has a well-established track record for infectious diseases and cancer, given its genetic malleability, safety and ability to induce strong T cell responses without the need for an adjuvant95,96. Some viral vectors, such as Ad5 and ChAd, usually need to be administered only once for protection and have natural tropism for the respiratory mucosa, which means they are amenable to respiratory mucosal vaccination79. The technology already exists for their large-scale clinical grade production and storage.
Thus, recombinant viral vectors are the second most common platform for COVID-19 vaccine development, with 4 candidates currently in clinical trials (Table 2), 38 under preclinical development5 and 3 (ChAdOx1 nCoV-19, Ad26-S and VSV-S) selected for US Operation Warp Speed97 (Table 2). The non-replicating viral platforms are mostly based on Ad5 or MVA, and most of these vaccine candidates express the S protein or RBD of SARS-CoV-2. Replication-competent viral vectors are mainly based on the vaccine strains of other human pathogens (such as measles or influenza viruses) or veterinary pathogens (such as vesicular stomatitis virus (VSV)). However, it will be important to consider whether humans have pre-existing immunity against the viral backbone (Table 1). Pre-existing antibodies can impair the ability of such vaccines to engage the immune system. Use of viral backbones such as ChAd, for which humans have little to no pre-existing immunity, can help to circumvent this issue79.
Ad5-nCOV, which is being developed by the Chinese vaccine company CanSino Biologics, is designed to induce neutralizing antibodies to SARS-CoV-2 S protein following intramuscular injection (Table 2). Without published preclinical data, it entered phase I/II clinical trials with three doses of vaccine tested98,99. Of note, these doses are 10–30 times higher than those used in previous trials of intramuscular vaccines100,101,102. Whereas the highest dose generated unacceptable toxicity and was dropped from the phase II study99, the smaller doses induced S protein-specific neutralizing antibodies in only 50% of the vaccine recipients98. The phase II study largely reaffirms the phase I observations that, although the vaccine induces both antibody and T cell responses, its potency is reduced by pre-existing immunity to Ad5, particularly in elderly participants99. Depending on geographical region, 35–95% of humans have significant circulating levels of neutralizing antibodies to Ad5 (ref.103). This is consistent with the rapidly declining antibody titres observed in a phase II Ad5-Ebola vaccine study104. The vaccine is entering further advanced trials in China and Canada, but the efficacy of this strategy is now in question105. Another human adenovirus-based COVID-19 vaccine, known as Ad26-S, is being developed by Johnson & Johnson, although there is still 40% seroprevalence for Ad26 in humans106. As Ad26 is inherently less immunogenic than Ad5 (ref.107), effective immunity requires repeated homologous or heterologous vaccination, as has been shown in Ad26-HIV and Ad26-Ebola vaccine studies in humans108,109. Nevertheless, a single parenteral administration of an Ad26-vectored COVID-19 vaccine (Ad26.COV2.S) offered robust protection in a non-human primate model of SARS-CoV-2 (ref.110).
ChAdOx1 nCoV-19 (also known as AZD-1222), which is being developed by Oxford University, UK, and AstraZeneca, is the most clinically advanced COVID-19 vaccine (Table 2). Humans have low seroprevalence for ChAd, hence its strong immunogenicity and utility for heterologous prime–boost COVID-19 vaccination79,107,111. The development of ChAdOx1 nCoV-19 is based on promising human studies with ChAdOx1-MERS vaccine112 and ChAdOx1-TB vaccine113. However, although intramuscular delivery of ChAdOx1 nCoV-19 reduced SARS-CoV-2 viral load in the lungs and prevented pneumonia in rhesus macaques, it did not reduce viral loads in the upper respiratory tract114. A recently reported phase I/II study shows its safety and the induction of potent neutralizing antibody and T cell responses following a single parenteral injection, which are boosted further by a second homologous vaccination115. It remains unclear from this trial to what extent both CD4+ and CD8+ T cell subsets were activated.
VSV-S is a replication-competent COVID-19 vaccine under development by Merck116 and other groups. Merck’s vaccine is built upon the licensure of its highly efficacious VSV-Ebola vaccine, which induces neutralizing antibodies and cellular immunity against Ebola virus surface glycoprotein117. VSV is a veterinary virus to which humans have no pre-existing immunity. However, the cloning capacity of the VSV vector is limited to 4 kb, and its suitability for respiratory mucosal vaccination is unclear. A single parenteral vaccination with a VSV vector expressing S protein provides protection against SARS-CoV-2 in both mouse and hamster models118,119. Among other viral-vectored candidates is non-replicating MVA. MVA has widely been explored as a vaccine carrier and has a cloning capacity of up to 30 kb. However, as it is not robustly immunogenic, MVA is often used as a booster vaccine or repeated injection is required to be effective, as was the case in clinical testing of an MVA-MERS-S vaccine120.
Inactivated viral vaccines
Physically or chemically inactivated viruses have been used successfully in human vaccines against polio, hepatitis A and influenza121,122. Inactivated viruses can be rapidly generated and scaled up in a pandemic situation using well-established infrastructure and methods123. Inactivated viral vaccines have few safety concerns, unlike their live attenuated counterparts, and they express a wide range of native viral antigens, including surface antigens with retained epitope conformations that can induce conformation-dependent antibody responses124,125.
Currently, there are five early clinical trials to assess inactivated SARS-CoV-2 vaccines (Table 2), with an additional nine candidates in preclinical development5. PiCoVacc, an inactivated SARS-CoV-2 and alum-adjuvanted vaccine developed by Sinovac Biotech Ltd in China, is the most advanced candidate with published preclinical results126. It protects rhesus macaques against SARS-CoV-2, with reduced viral titres and immunopathology associated with antibodies to S protein and nucleocapsid126. BBIBP-CorV, another inactivated virus candidate, which is being developed by Chinese state-owned Sinopharm, was tested in a range of animal models, with demonstrated efficacy in non-human primates127. Although these findings provide optimism, the observations were made in rather short-term studies and should be interpreted with caution.
Inactivated viral vaccines often require an adjuvant and repeated administration to be effective (Table 1). The use of alum as an adjuvant126,127 makes them unsuitable for respiratory mucosal delivery128. Although the protection mediated by intramuscular immunization with PiCoVacc or BBIBP-CorV indicates some level of mucosal immunity, probably through the transport of systemic antibodies to the lungs, the durability of such immunity remains unclear as SARS-CoV-2 challenge was performed 1–4 weeks after vaccination126,127. Furthermore, similarly to protein subunit vaccines, inactivated viral vaccines are poor inducers of cytotoxic CD8+ T cells, which are likely to be required for an effective COVID-19 vaccine.
Studies with inactivated SARS-CoV and respiratory syncytial virus vaccines have reported vaccine-related enhancement of disease, likely involving a TH2 cell response and lung eosinophilia, which may be worsened in aged hosts56,74,129. Although PiCoVacc or BBIBP-CorV did not worsen lung disease within 7 days after infection, alum is known to drive TH2 cell-mediated immune responses, which warrants further safety investigations. The use of TH1 cell-skewing modified alum or other adjuvants such as CpG may avert such safety concerns130,131.
Protein subunit vaccines
Currently, there are seven COVID-19 subunit vaccines in clinical trials (Table 2), with 50 other candidates under preclinical development, making this the most common platform5. Subunit vaccines primarily induce CD4+ TH cell and antibody responses. Therefore, most of these vaccines contain full-length SARS-CoV-2 S protein or portions of it with the goal of inducing neutralizing antibodies, similarly to the majority of SARS and MERS vaccines, which had differing levels of efficacy132,133,134.
Subunit vaccines can be designed to focus the immune response towards neutralizing epitopes, thereby averting the production of non-neutralizing antibodies that may promote ADE of disease135. However, unlike nucleic acid-based or viral-vectored vaccines, recombinant S proteins in subunit vaccines could have an improper epitope conformation unless they are produced in mammalian cells136. Proteins or peptides alone are poorly immunogenic and generally require not only an adjuvant but also repeated administration, and they are poor activators of CD8+ T cell responses (Table 1). Furthermore, this platform is generally unsuitable for respiratory mucosal vaccination. As is the case for inactivated viral vaccines, use of unmodified alum as an adjuvant skews the immune response towards TH2 cell-like responses56, which is undesirable for host defence against SARS-CoV-2 and may have a role in ADE of disease74,130. In this regard, subunit COVID-19 vaccines being developed by GlaxoSmithKline and Novavax use AS03 and Matrix-M adjuvants, respectively5.
Virus-like particles
VLPs are spontaneously forming particles composed of several structural viral proteins that are co-expressed or admixed. Several commercial vaccines, such as hepatitis B and human papillomavirus vaccines, are based on VLPs137. In the case of enveloped coronaviruses, VLPs form when the viral proteins S, M and E, with or without N, are co-expressed in eukaryotic producer cells138,139. This results in active budding from the producer cells of VLPs that are structurally identical to the infectious virus but lack the viral genome and thus are non-infectious. The presence of S protein on the surface of VLPs enables them to bind and enter ACE2+ cells in the same manner as the parent virus140. Unlike subunit vaccines, the array of S protein on the VLP surface crosslinks the B cell receptor and directly activates B cells, but, like subunit and inactivated viral vaccines, VLPs also typically require an adjuvant and repeated administration137. Notwithstanding this, the VLP technology is well established, the biology and safety of coronavirus VLPs are understood and their large-scale production to Good Manufacturing Practice standards is relatively straightforward.
Currently, there is only 1 VLP-based COVID-19 vaccine in clinical trials (Table 2), with 12 more under preclinical development5. These are produced either in vivo from a viral vector, such as MVA, that expresses the VLP components (a platform being developed by GeoVax) or more often in vitro from producer cells. Notably, Medicago, a Canadian company, produces its SARS-CoV-2 VLPs from genetically engineered plants. Its unpublished results seem to suggest efficacy in inducing neutralizing antibodies in mice141.
Nucleic acid-based vaccines
Recombinant plasmid DNA has been explored as a vaccine platform for decades, whereas mRNA has emerged more recently as a promising platform142,143. Currently, there are 6 mRNA-based COVID-19 vaccines and 4 DNA-based COVID-19 vaccines in clinical trials (Table 2), with 27 such vaccines (16 mRNA-based and 11 DNA-based vaccines) under preclinical development5.
The antigen-encoding mRNA complexed with a carrier such as lipid nanoparticles can be efficiently delivered in vivo into the cytoplasm of host cells for protein translation and post-translational modifications142,144, which is an advantage over recombinant protein subunit vaccines. mRNA vaccines are non-infectious and are synthesized by in vitro transcription, free of microbial molecules. These beneficial features differentiate mRNA vaccines from live attenuated viral vaccines, inactivated viral vaccines, subunit vaccines and recombinant viral-vectored vaccines in terms of safety, efficacy and issues of antivector immunity, enabling their rapid and inexpensive production and repeated vaccination142 (Table 1).
mRNA-1273, which is produced by Moderna, an American biotech company that has experience with mRNA-based MERS vaccines, encodes a prefusion-stabilized SARS-CoV-2 S protein encapsulated in lipid nanoparticles. It entered clinical testing even before the release of preclinical data145. Recently published phase I clinical trial data indicate that low and medium doses of two repeated parenteral injections are generally safe and induce strong S protein-specific antibody responses and a primarily CD4+ T cell response in most trial participants146. Pfizer and BioNTech are also assessing an mRNA–lipid nanoparticle vaccine encoding the S protein RBD (known as BNT162b1) in humans, who developed robust S protein-specific antibody and CD4+ and CD8+ T cell responses following two repeated parenteral injections147,148. The Pfizer/BioNTech and Moderna vaccines have both been selected for US Operation Warp Speed97 (Table 2).
Although no mRNA vaccine has yet been licensed for human use, their potential is supported by previous studies of influenza, rabies and Zika virus infections in animals149,150,151,152,153. For example, an mRNA vaccine for influenza virus induced long-term humoral immunity in young and aged mice149, and an mRNA vaccine for Zika virus induced both antibodies and cytotoxic CD8+ T cells in mice154. However, two clinical studies show disparities in the magnitude and longevity of immune responses induced by mRNA vaccines152,155. Thus, although mRNA-based COVID-19 vaccines show promise from early clinical testing, questions remain about their protective efficacy in humans. It is also unclear whether mRNA vaccines are amenable to respiratory mucosal delivery.
Plasmid DNA vaccines share several characteristics with mRNA vaccines, including safety, ease of production and scalability156. However, they are poorly immunogenic, requiring multiple doses and the addition of an adjuvant. Currently, there are four plasmid DNA-based COVID-19 vaccines in clinical testing (Table 2), with 11 more under preclinical development. INO-4800, a plasmid DNA vaccine expressing SARS-CoV-2 S protein, is being developed by the US biotech company Inovio Pharmaceuticals. A preclinical study in mice and guinea pigs examined the immunogenicity of this vaccine but did not provide any data pertaining to protection against challenge157. Two repeated injections of an S protein-expressing plasmid DNA vaccine resulted in robust protective immunity in rhesus macaques158.
Conclusions and outlook
The world is in dire need of safe, effective COVID-19 vaccine strategies. Many laboratories and companies have scrambled to rapidly develop these vaccines, resulting in more than 160 vaccine candidates, with a handful having entered phase I, II and III clinical trials within a short period of 6 months. Although we are just beginning to understand COVID-19 and its vaccine requirements, most of the advanced vaccine platforms have been extensively explored for other infections and cancer79,95,96,159. While it is important to pursue various vaccine strategies in parallel, it is equally important not to lose sight of this existing scientific knowledge to make well-informed decisions around which strategies to prioritize.
The various vaccine platforms and strategies have their immunological pros and cons (Table 1), but modern immunological principles and data from prior studies of similar platforms lead us to surmise that a parenteral COVID-19 vaccine strategy capable of inducing a robust, durable response involving both neutralizing antibodies and T cells should provide a significant level of protection. Almost all of the current vaccines in the human immunization programme are delivered via the skin or muscle, and most of the current COVID-19 vaccine strategies also focus on the parenteral route of vaccination (Table 2). We further surmise that a respiratory mucosal vaccine strategy capable of inducing these responses directly in the respiratory mucosa will be most effective in the early control or clearance of SARS-CoV-2. This is particularly relevant to high-risk elderly populations, who will require a particularly robust vaccine strategy. In this regard, a respiratory mucosal vaccine strategy for COVID-19 may draw on the successful experience in respiratory mucosal delivery of influenza, measles and TB vaccines to humans160,161,162. Respiratory mucosal vaccination also has the advantages of being needle-free and requiring a much smaller dose than the parenteral route. However, compared with the parenteral route, fewer vaccine platforms are safe and effective for respiratory mucosal vaccination. Furthermore, the use of inhalational devices for respiratory mucosal delivery may potentially be a limiting factor for widespread application in resource-poor settings.
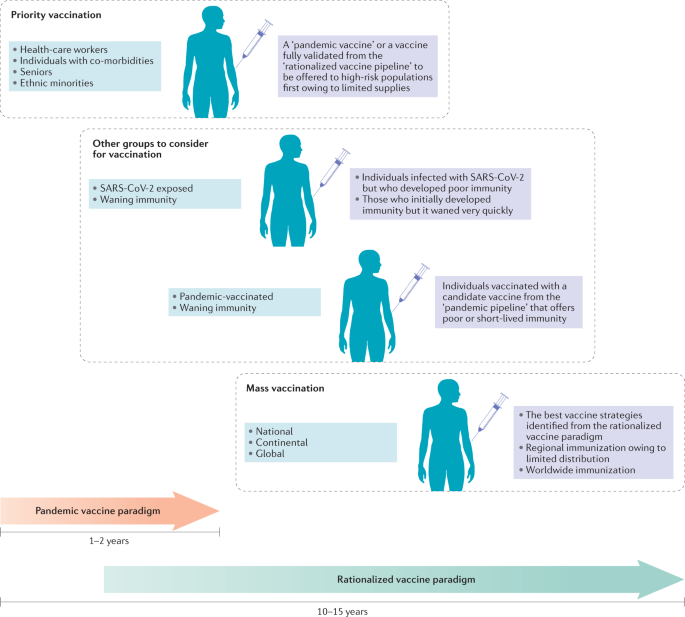
According to the pandemic vaccine development paradigm (Fig. 2), the conventional vaccine development milestones are compressed from a time frame of 10–15 years to 1–2 years, with overlapping preclinical, clinical and scale-up manufacturing processes occurring in parallel6. Owing to the accelerated development process, the interim data from ongoing clinical and preclinical vaccine studies are being published almost in real time. As a result, crucial information about the longevity and quality of vaccine-induced protective immunity is unavailable. As transmission rates and the numbers of new cases have reduced in many countries, it is uncertain whether the phase II and phase III studies of the front-runner candidates will reach a reliable conclusion with regard to their protective efficacy. Furthermore, these vaccine candidates have been studied in isolation, which makes it difficult to directly compare the effectiveness of different candidates. Thus, it would be premature to hail the safety and immunogenicity observed in COVID-19 vaccine trials as a real success. To a large extent, such outcomes could be anticipated from past studies testing the same platforms and delivery routes. Nevertheless, rapid deployment of a vaccine with preclinical efficacy data but limited clinical data to high-risk populations may be necessary (Fig. 2).
In response to the urgent demand for a vaccine, more than two dozen candidate vaccines are advancing through clinical trials following an expedited pandemic vaccine development paradigm, with many steps of the development process occurring in parallel before a successful outcome of previous steps has been confirmed. Vaccine candidates will continue to be preclinically and clinically evaluated following conventional and/or rationalized vaccine development processes over the next few years. These efforts will evolve to meet the demands for vaccination in several likely scenarios that are predicted on the basis of sociopolitical challenges and the emerging data regarding the trajectory of the coronavirus disease 2019 (COVID-19) pandemic and the host response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). One scenario is the priority vaccination of high-risk populations such as health-care workers, seniors, people with co-morbidities and ethnic minorities, who have been disproportionately affected by COVID-19, when vaccine supply is initially limited. Aside from these prioritized groups, it may also be necessary to consider that asymptomatic individuals, patients who have recovered from COVID-19 but generated poor immunity or whose immunity quickly waned, and individuals who received a rapidly developed ‘pandemic’ vaccine that provided suboptimal protection or rapidly waning immune responses may require a booster vaccination to ensure sufficient levels of population protection for herd immunity. Ultimately, regional, continental and global populations will be subject to mass vaccination programmes based on the extent of national and global vaccine distribution and also likely according to the relative regional severity of outbreaks.
The evolving process of vaccine development will continue over the next few years until more clinical trials are completed, additional vaccine strategies are evaluated and host defence against SARS-CoV-2, including postinfection immunity, is better understood (Fig. 2). Probably not until then will global mass immunization become a reality. It is possible that the populations that receive the first round of vaccines will have waning immunity and require boosting using improved second-generation COVID-19 vaccines. Furthermore, in addition to unexposed individuals, some individuals who have recovered from COVID-19 who develop poor or waning immunity may also require vaccination163.
Given the challenges in resources, manufacturing and issues associated with distribution and regional protectionism, the implementation of vaccination programmes will likely be uneven, asynchronous and variable — involving different vaccine platforms and strategies around the globe164,165. In this regard, some resource-rich countries have already secured large numbers of doses of different candidate vaccines without knowing which one may prove effective. The heated debate has begun globally over who should be at the front of the line when vaccine supply is limited. The founding of the COVID-19 Vaccines Global Access (COVAX) Facility by Gavi, the Coalition for Epidemic Preparedness Innovations (CEPI) and the WHO is an attempt to garner resources and unite higher- and lower-income countries for the coordinated, rapid, transparent and equitable access to COVID-19 vaccines worldwide.


No comments:
Post a Comment
Comments always welcome!