Researchers to begin human trials for promising new inhaled COVID-19 vaccines designed to combat variants of concern
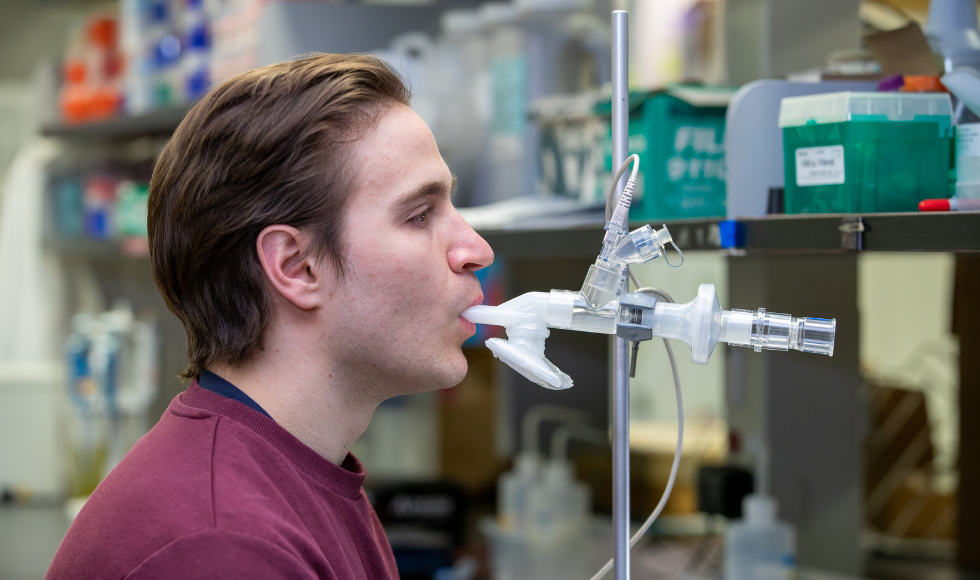
Researcher Michael D’Agostino demonstrates use of the nebulizer.
DECEMBER 7, 2021
Update, March 3, 2022: Human clinical trials for these vaccines have begun and are ongoing. This story was originally published on Dec. 7, 2021.
Human trials are set to begin for two next-generation COVID-19 vaccines developed by a team of scientists at McMaster University.
Both are designed to combat variants of concern and will be delivered by inhaled aerosol, not by injection, and will target the lungs and upper airways, where respiratory infections begin.
These are two of the very few COVID-19 vaccines being developed in Canada. Phase 1 of the clinical trials was recently approved by Health Canada.
Study participants who have previously received two doses of a COVID mRNA vaccine, such as BioNTech Pfizer or Moderna, will receive a booster as part of the clinical trial.
As the pandemic transitions into an endemic phase, researchers around the world have been racing to develop more efficient vaccines to follow the first generation of emergency vaccines that were designed to stem the initial spread of COVID-19.
The new McMaster vaccines, which express three different SARS-CoV-2 proteins, including the distinctive spike protein, are designed to also target other parts of the virus that do not change or mutate. Activating the immune system against three different proteins, rather than one, should provide better protection against variants of concern, the researchers say.
“It is critical to continue research on new forms of COVID vaccines that work in a different way and could be used to boost immunity in people who have already been vaccinated,” says Fiona Smaill, a professor of Pathology and Molecular Medicine at McMaster who is leading the clinical trial.
“By targeting a breadth of immune responses to different parts of the COVID virus, we expect to see broader protection,” she says.
Meet the researchers behind McMaster’s inhaled COVID-19 vaccines
The inhaled delivery concept draws on two decades of research and development on a tuberculosis vaccine led by Zhou Xing, a co-principal investigator and professor in the Department of Medicine and McMaster Immunology Research Centre.
“Our vaccine strategy differs from all of the current first-generation COVID-19 vaccines in the route of delivery. Ours gets delivered into the lung via inhaled aerosol to induce respiratory mucosal immunity, known to provide best protection against respiratory pathogens,” Xing says.
Researchers will examine how the immune response develops in the lungs and blood after vaccination and monitor for possible side effects.
Researchers are comparing two strains of weakened adenovirus as platforms for the vaccines. In their natural form, adenoviruses cause respiratory infections such as the common cold, and in rare cases can cause a lung infection such as pneumonia. In their weakened form they do not spread disease, but can be customized to serve as vehicles, or vectors, to trigger targeted immune responses.
“McMaster has a very long and illustrious history in the study of adenoviruses. This human trial builds on that pioneering work, and in addition to addressing an issue of tremendous public health importance, will also advance our fundamental understanding of how to use these viruses most effectively as vaccine vectors,” says Matthew Miller, an associate professor with McMaster’s Michael G. DeGroote Institute for Infectious Disease Research and co-principal investigator.
The vaccines were produced at McMaster’s Robert E. Fitzhenry Vector Laboratory, one of a very few facilities in Canada with the capacity to develop and produce viral vector vaccines for clinical trials. The laboratory and the researchers are part of McMaster’s Global Nexus for Pandemics and Biological Threats.
“The Fitzhenry Vector lab is a unique resource which made it possible for our team to take innovative research all the way from concept to manufactured vials of vaccine available to boost human volunteers,” says Brian Lichty, an associate professor in the Department of Medicine who is co-leading the vaccine development.
Virologist and Vice-President, Research, Karen Mossman, says the combination of McMaster’s pioneering research, world-class talent and state-of-the-art infrastructure uniquely positions the university to help Canadians during this pandemic and future health crisis.
“I’ve had a front-row seat to this made-in-Canada solution and it’s game-changing for COVID vaccine delivery,” she says. “We’re home to some of the country’s – indeed, the world’s – foremost researchers who work at the intersection of basic and applied research, ensuring their work is translated into products and technologies that advance societal health and well-being.”
At least 30 healthy volunteers will take part in the study, which is funded by the Canadian Institutes for Health Research. If Phase 1 is successful, the team has manufactured sufficient vaccine doses to move forward with much larger clinical trials, which could potentially lead to broader use, the researchers say.
About Canada’s Global Nexus for Pandemics and Biological Threats, McMaster University
Building on a world-leading track record of infectious disease research, Canada’s Global Nexus at McMaster University brings together a vast, international network of researchers, government, industry, health care and other partners with the goal of finding solutions to the current pandemic, while preparing for future global health threats.
No comments:
Post a Comment
Comments always welcome!